Introduction
In common language, the term “polymer” elicits references to many ordinary, practical items, including the phrase “paper or plastic” in checkout counters. “Polymer” is derived from Greek, with “poly” meaning many and “mer” meaning part. For example, in typical human-made polymers such as polyethylene, polyurethane, and polyvinyl chloride, there are many units of either ethylene, urethane, or vinyl chloride put together to form the ultimate polymer. In all commonly known polymers, the repeating units are made from mainly carbon-containing units. Hence, they all belong to the group of organic polymers. In addition to human-made organic polymers, nature is abundant with organic polymers such as wool, silk, proteins, starch, and cellulose. In contrast, any material that does not contain the element carbon can be considered “inorganic.”
Borates, silanes, and polyphosphazenes are inorganic polymers since they do not contain carbon. Typically, organic polymers are flexible and have an operating window with an upper bound of 400 oC. For example, the polyimide film Kapton has an operating window of –269 oC to 400 oC. Carbon-containing polymers with high thermal and oxidative stabilities are stiffer materials, such as the rigid rod polymer poly(benzobisoxazole) (Zylon). In such polymers, the in-plane aromatic conjugation restricts the availability of functional groups that can be attacked by oxygen. More flexible polymers like polyethylene, polypropylene, and polystyrenes tend to be attacked by oxygen at much lower temperatures and have melting temperatures below 250 oC. In contrast, inorganic polymers are usually rigid and tend to have higher operating windows. To combine the properties of organic and inorganic polymers, a new group of polymers emerged that comprised both groups. Thus, inorganic-organic hybrid polymers are a rarified class of materials possessing combined properties of both inorganic main group element- and carbon-containing components.
The terms “inorganic-organic” or “organic-inorganic” were first coined in 1990 by Saegusa and Chujo, who reported the polymer poly(N-acetylethylenimine)(polyoxazoline) with terminal triethoxysilyl groups [1]. In addition to increasing the flexibility of polymers, a principal intent in forming an inorganic-organic hybrid polymer is to improve its thermal and oxidative properties. One way to circumvent the oxidation problem is by slowing down the diffusion of oxygen into a polymersample’s interior during oxidation, i.e., by diffusion-limited-oxidation (DLO) [2]. The most common polymers that can provide such a condition are silanes and siloxanes. In addition to the flexibility of their backbones, they tend to form inorganic silica (SiO2, melting point [M.P.] = 1710 oC) during oxidation that significantly impedes the diffusion of oxygen. Other groups of inorganic polymeric entities that provide such DLO conditions contain the elements aluminum, boron, and phosphorous. During oxygen attack, these polymers produce alumina (Al2O3, M.P. = 2072 oC), boron oxide (B2O3, M.P. = 450 oC), and phosphorus pentoxide (P2O5, M.P. = 340 oC) during oxidation, which substantially slows the diffusion of oxygen. In general, inorganic-organic polymers are used in coatings, functional particles, bulk materials, fibers, and composites [3]. Such applications are crucial for the Navy.
Siloxane Polymer Origins
The origins of siloxane chemistry can be attributed to the English chemist Frederic Kipping and his coworkers in the early 1900s. However, the discoverers of siloxanes did not deem this group of compounds to be of significant interest, as evident in the statement of Kipping during his 1936 Bakerian lecture that “the prospect of any immediate and important advance in this section of organic chemistry does not seem to be very hopeful” [4, 5]. Fortunately, Kipping was proven to be wrong by a renaissance in siloxane chemistry in the 1940s led by Hyde and Delong at the Corning Glass Works [6], McGregor and Warrick at the Mellon Institute [7], and Rochow at the General Electric Company [8].
During this time, the synthesis of polysiloxanes was perfected and led to other silarylene-siloxane polymers and silalkarylene-siloxane polymers in the 1950s. These were the original inorganic-organic hybrid polymers, although the term was not coined at the time of their conception. Even though they were high-performance elastomeric materials, a major development occurred in the 1960s and 1970s with the discovery of carborane-siloxane polymers [9, 10]. (Carboranes are three-dimensional compounds of boron, carbon, and hydrogen with polyhedral skeletons of the general formula CpBqHp+q.) The carborane-siloxane polymers possessed exceptional thermal and oxidative stabilities. However, they were mainly inorganic polymers and thermoelastomeric materials like siloxane polymers.
Inorganic-Organic Hybrid Polymers’ History at the Naval Research Laboratory’s (NRL’s) Chemistry Division
NRL Chemistry Division’s entry into this exciting area of polymeric materials occurred in the 1990s. To add to the suite of their promising high-performance phthalonitrile polymers with the intent to develop novel inorganic-organic hybrid and “thermosetting” polymeric versions of the existing carborane-siloxane polymers, Henderson and Keller reported the original synthesis of poly(carborane-siloxane-acetylene)s (PCSA) with exceptional thermal and oxidative stabilities [11]. In Figure 1, the organic entity in this group of polymers was derived by the dechlorination of hexachlorobutadiene (2) using n-butyl lithium yielding the dilithiated diacetylene entity (3) which, upon reaction with the halogenated carborane-siloxane monomer (named DEXIL monomer[4]), produced PCSA (1). Containing about 10 inorganic repeat units linked by the organic diacetylene entities, these oligomers retained up to 85% and 92% weight upon pyrolysis to 1000 oC in a nonoxidizing and oxidizing environment. This polymer started to crosslink from around 250 oC, with an exotherm peaking around 350 oC (Figure 1), by either 1,2- or 1,4- polymerization of the diacetylene groups to yield thermosetted carbon domains.
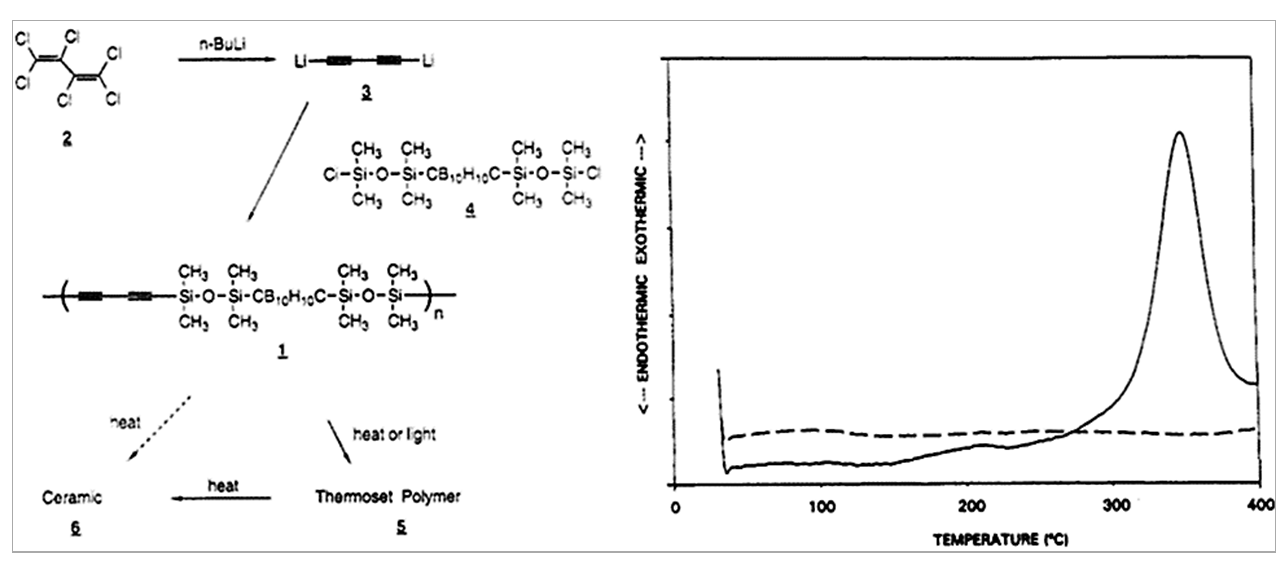
Figure 1. (Left) Reaction Scheme for the Synthesis of Poly(Carborane-Siloxane-Acetylene) (PCSA) and (Right) the Differential Scanning Calorimetry Thermogram of Neat PCSA (Solid) and Cured PCSA (Dashed) (Source: Reproduced With Permission From the American Chemical Society).
Subsequently, the carboraneless version of this polymer was also produced within the year by Son and Keller [12]. This was followed by the synthesis of a block copolymer wherein blocks of PCSA were alternated with siloxane-diacetylene monomers [13]. At this point, hydrosilylation was used as a reactive means to produce an acetylene-containing silicon (Si)monomer which could be further reacted with other aromatic organic entities to produce new versions of PCSA [14].
To fundamentally understand the thermo-oxidative stability of PCSA, surface analysis studies were carried out by Pehrsson et al. using scanning electron microscopy, X-ray photoelectron spectroscopy, scanning Auger microprobe scattering, and Raman microprobe scattering [15]. It was seen that PCSA samples heated to 400 oC in argon exhibited no inorganic segregation; however, treatment to the same temperature in air produced surface layers of boron and Si oxide. Furthermore, samples annealed in argon to 900 °C and then oxidized at 500 °C for up to 100 hr grew a continuous Si oxide surface layer with almost no underlying boron oxide. This layer retarded oxidation of the bulk sample at 500 oC. Thus, the thermo-oxidative stability was determined to be from the DLO of the polymer, with the formation of SiO2-, B2O3-, and the possible formation of borosilicate-containing protective barriers at higher temperatures.
Further, in continuing inorganic-organic hybrid polymer research, a PCSA-like polymer wherein the carborane clusters were substituted by a clusterless B-Ph group was produced by Sundar and Keller [16]. The thermal stability of this polymer up to 1000 oC in nitrogen was found to be lower than PCSA (72.1% vs. 85%). However, the crosslinked versions exhibited similar oxidative stabilities as those obtained from PCSA. The main advantage of this polymer is substituting the expensive carborane with the inexpensive B-Ph group, thus reducing overall cost.
Subsequently, Bucca and Keller attempted to incorporate an aromatic group-like phenyl(benzene) in the backbone of the carborane-siloxane inorganic-organic hybrid polymer [17]. A 4-phenylethynylphenyl unit was used to introduce the aromatic group. However, the presence of a labile phenyl group after crosslinking caused the polymer to have low thermal stability. Further, the ligand 1,4-bis(dimethylchlorosilyl)benzene was used to incorporate a benzene(phenyl) group into a series of polymers with thermal stabilities between 79% and 86% [18].
An interesting development in the progress of inorganic-organic hybrid polymers occurred in 1998 when Houser and Keller introduced ferrocene in the PCSA polymer in the form of bridging groups between siloxane-carborane-siloxane-diacetylene entities [19]. This polymer, producing iron (Fe)-containing ceramic had a weight retention of 78% at 1000 oC in nitrogen compared to the typical ferrocenyl-siloxyl polymers with a weight retention of around 50% [20]. The DEXIL monomer was further used in hydrosilylation reaction with poly(methylhydrosiloxane) to produce crosslinked networks with high stability [21]. Oxidation-resistant thermosets were also formed from thermal copolymerization of acetylenic monomers containing boron and Si [22] and diacetylene-siloxane-carborane ceramic precursors [23].
In the early 2000s, a new set of high-temperature elastomers was synthesized at NRL from silarylene-siloxane-diacetylene linear polymers by Homrighausen and Keller [24, 25]. These differed from the silarylene-siloxane of the 1950s in that they contained the crosslinking unit diacetylene, which enabled conversion to a thermoset. Furthermore, both the diacetylene and phenyl groups were incorporated in the backbone of an inorganic-organic hybrid polymer using hydroxy-terminated, oligomeric poly(silarylene disiloxane)s via rhodium-catalyzed dehydrogenative coupling for their use in the aminosilane-disilanol polymerization reaction [26].
In the meantime, the practical implications of PCSA and other produced inorganic-organic variants were becoming obvious as Keller in 2002 demonstrated that they could protect carbon fibers (CFs) from oxidation when applying PCSA as a protective coating [27]. While uncoated CFs were found to catastrophically degrade between 600 oC and 800 oC, PCSA-coated CFs retained up to 96 wt% when heated in air to 1000 oC. This was truly impressive! Around that time, Beckham and Keller synthesized diacetylene-terminated diacetylene containing polysiloxanes—a new addition to the class of polysiloxanes [28].
The research in the early 2000s on PCSA and its derivatives hinged on making these polymers elastomeric. Kolel-Veetil and Keller explored two ways of effecting this [29]. In the first method, the concentration of diacetylene units was diluted in PCSA to impart elasticity to the polymers. In the second method, different kinds of block polymers were incorporated in the PCSA and its variants, and their elastomeric properties as a function of the glass transition temperatures and corresponding thermal stabilities were evaluated [30].
Furthermore, Kolel-Veetil et al. expanded the utility of these polymers to produce transition metal (TM)-derived nanoparticles (NPs) by reacting TM complexes with the diacetylene groups in PCSA to obtain molecular-level functionalization [31]. In reacting PCSA with the TM complex, Cp2Mo2(CO)6, functionalization of the diacetylene occurred, and the TM-PCSA complexes on pyrolysis produced a superconducting mixture that contained β-Mo2C NPs and carbon nanotubes. In a seminal paper that garnered the prestigious Berman Publication Award of NRL, Kolel-Veetil et al. demonstrated that by controlling the rate and temperature of pyrolysis, one could produce different phases of Mo2C NPs and therefore different conductivity properties for the mixture [32]. Thus, on pyrolysis only to 850 oC, smaller (~2–4 nm)-sized α-Mo2C NPs were formed. Due to the sizes being below the Anderson criterion limit, these NPs were unable to sustain superconductivity in a typical BCS system since the coherence length of the Cooper pairs was larger than the particle sizes [33]. When pyrolysis was performed up to 1000 oC, β-Mo2C of larger sizes was produced that sustained superconductivity with a Tc of ~5 oC (Figure 2).
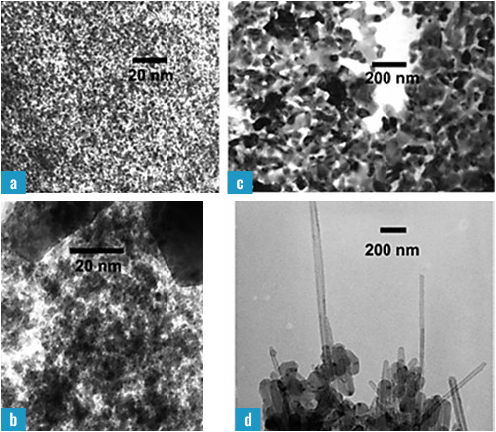
Figure 2. TEM Micrographs of the 850 °C Pyrolysis Product Containing Predominantly (a) α-Mo2C NPs and (b) α-Mo2C NPs With a Few β-Mo2C NPs; and the 1000 °C Pyrolysis Product Containing (c) Larger β-Mo2C NPs and (d) Larger β-Mo2C NPs With MWCNTs (Source: Reproduced With Permission From the American Chemical Society).
In a similar vein, Kolel-Veetil et al. used a ferrocene-containing, siloxane-diacetylene polymer like the one developed in 1998 [34]. They showed that depending on the rate of thermal treatment, one could either form Fe NPs with CF and Si carbide or have carbon nanocapsules sequester both Fe and Si during reaction to produce the ferromagnetic Fe5Si3 NPs.
In 2009 and 2012, Kolel-Veetil et al. utilized the hydrosilation reactions to make new inorganic-organic hybrids of polyoctahedral silsesquioxanes (POSS), the smallest unit of silica [35, 36]. In the first example, crosslinked dendritic networks of POSS units with diacetylene crosslinkers were synthesized [35]. In the second instance, crosslinked dendritic networks of POSS units with acetylene crosslinkers were synthesized [36]. The latter allowed the production of α-cristobalite in the converted ceramic.
Finally, in 2013, Kolel-Veetil et al. were able to produce an inorganic-organic polymer variant of PCSA, known as m-poly(carborane-siloxane-arylacetylene) (m-PCSAA) and p-poly(carborane-siloxane-arylacetylene) (p-PCSAA) by incorporating p-diethynylbenzene and m-diethynyl-benzene (Figure 3) [37]. These variants have slightly higher thermal and oxidative stabilities than PCSA. Impressively, they also protect high-performance organic fibers such as Kevlar, Zylon, and electrically conducting wires during operation at high temperature and voltage. Furthermore, these polymers also have exceptional dielectric properties.

Figure 3. (Top Box) The In-Backbone Aromatic Group-Containing Carboranylenesiloxanes m-PCSAA and p-PCSAA and Their Diacetylene Counterpart, PCSA. (Bottom Box) Synthetic Schemes for m-PCSAA and p-PCSAA (Source: Reproduced With Permission From the American Chemical Society).
Today’s Polymers
From 2013 to the present, various materials properties of these polymers have been explored that have created some exciting applications. In 2020, PCSA, m-PCSAA, and p-PCSAA were licensed by the commercial entity Boron Specialties, Inc. in Ambridge, PA. Many more exciting future applications are also being created for these inorganic-organic hybrid polymers.
Conclusions
While the science of siloxanes has come a long way since their discovery, the advent of inorganic-organic hybrid polymers has opened new possibilities for further evolution. The ultimate utility of the inorganic entities in the inorganic-organic hybrid siloxane polymers has been manufacturing DLO-producing oxide surfaces. The organic groups function as crosslinking sites enabling the creation of thermosets and sites for TM-functionalization and yielding a very impressive suite of TM-containing compositions with exceptional conducting and magnetic properties. Such developments have enabled the production of novel coatings, functional particles, bulk materials, fibers, and composites that are very important to the Navy. Thus, further growth in the science of these polymers, contrary to Kipping’s trepidations, will only be limited by the imagination of the scientist.
Acknowledgment
The author gratefully acknowledges support by the NRL 6.1 Base program (FY23 Chemistry Division 6.1 Base Program; P.I.: M. K. Kolel-Veetil).
References
- Saegusa, T., and Y. Chujo. “An Organic/Inorganic Hybrid Polymer.” Journal of Macromolecular Science: Part A – Chemistry, vol. 27, nos. 13–14, pp. 1603–1612, 1990.
- Celina, M. C. “Review of Polymer Oxidation and Its Relationship With Materials Performance and Lifetime Prediction.” Polymer Degradation and Stability, vol. 98, no. 18, pp. 2419–2429, 2013.
- Haas, K.-H., and G. Schottner. “Hybrid Inorganic-Organic Polymers.” Encyclopedia of Glass Science, Technology, History, and Culture, Volume II, First Edition, Pascal Richet, pp. 1057–1068, 2021.
- Kipping, F. S. “The Bakerian Lecture: Organic Derivatives of Silicon.” Proceedings of the Royal Society of London A, vol. 159, pp. 139–148, 1937.
- Dvornic, P. R., and R. W. Lenz. High Temperature Siloxane Elastomers. Hüthig & Wepf Verlag Basel, Heidelberg, New York, 1990.
- Hyde, J. F., and R. C. Delong. “Condensation Products of the Organo-Silane Diols.” Journal of the American Chemical Society, vol. 63, pp. 1194–1196, 1941.
- McGregor, R. R., and E. L. Warrick. U.S. Patent 2,380,057, 1945.
- Rochow, E. G. “The Direct Synthesis of Organosilicon Compounds.” Journal of the American Chemical Society, vol. 67, pp. 963–965, 1945.
- Mayes, N., J. Green, and M. S. Cohen. Journal of Polymer Science, Part A-1, vol. 5, pp. 365–380, 1967.
- Peters, E. N. Journal of Macromolecular Science Review Macromolecular Chemistry, vol. C17, no. 2, pp. 173–182, 1979.
- Henderson, L. J., and T. M. Keller. “Synthesis and Characterization of Poly(Carborane-Siloxane-Acetylene).” Macromolecules, vol. 27, no. 6, pp. 1660–1661, 1994.
- Son, D. Y., and T. M. Keller. “Synthesis and Characterization of Linear Siloxane-Diacetylene Polymers.” Macromolecules, vol. 28, no. 61, pp. 399–400, 1995.
- Son, D. Y., and T. M. Keller. “Oxidatively Stable Carborane-Siloxane-Diacetylene Polymers.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 33, pp. 2969–2972, 1995.
- Son, D. Y., D. Bucca, and T. M. Keller. “Hydrosilylation Reactions of Bis(Dimethylsilyl)Acetylenes: A Potential Route to Novel σ– and π-Conjugated Polymers.” Tetrahedron Letters, vol. 37, no. 10, pp. 1579–1582, 1996.
- Pehrsson, P. E., L. J. Henderson, and T. M. Keller. “High-Temperature Oxidation of Carborane-Siloxane-Acetylene-based Polymer.” Surface and Interface Analysis, vol. 24, no. 3, pp. 145–151, 1996.
- Sundar, R. A., and T. M. Keller. “Synthesis and Characterization of Linear Boron-Silicon-Diacetylene Copolymers.” Macromolecules, vol. 29, no. 10, pp. 3647–3650, 1996.
- Bucca, D., and T. M. Keller. “Thermally and Oxidatively Stable Thermosets Derived From Preceramic Monomers.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 35, no. 6, pp. 1033–1038, 1997.
- Sundar, R. A., and T. M. Keller. “Linear Diacetylene Polymers Containing Bis(Dimethylsilyl)Phenyl and/or Bis(Tetramethyldisiloxane)Carborane Residues: Their Synthesis, Characterization and Thermal and Oxidative Properties.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 35, no. 12, pp. 2387–2394, 1997.
- Houser, E. J., and T. M. Keller. “Linear Ferrocenylene-Siloxyl-Diacetylene Polymers and Their Conversion to Ceramics With High Thermal and Oxidative Stabilities.” Macromolecules, vol. 31, no. 12, pp. 4038–4040, 1998.
- Patterson, W. J., S. P. McManus, and C. U. Pittman, Jr. Journal of Polymer Science, Polymer Chemical Education, vol. 12, pp. 837–848, 1974.
- House, E. J., and T. M. Keller. “Hydrosilation Routes to Materials With High Thermal and Oxidative Stabilities.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 36, no. 11, pp. 1969–1972, 1998.
- Armistead, J. P., E. J. Houser, and T. M. Keller. “Diacetylene-Siloxane-Carborane Thermosets and Ceramic Precursors.” Applied Organometallic Chemistry, vol. 14, no. 5, pp. 253–260, 2000.
- Bucca, D., and T. M. Keller. “Oxidation-Resistant Thermosets Derived From Thermal Copolymerization of Acetylenic Monomers Containing Boron and Silicon.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 37, no. 23, pp. 4356–4359, 1999.
- Homrighausen, C. L., and T. M. Keller. “High-Temperature Elastomers From Silarylene-Siloxane-Diacetylene Linear Polymers.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 40, pp. 88–94, 2002.
- Homrighausen, C. L., and T. M. Keller. “Synthesis and Characterization of a Silarylene-Siloxane-Diacetylene Polymer and Its Conversion to a Thermosetting Plastic.” Polymer, vol. 43, no. 9, pp. 2619–2623, 2002.
- Homrighausen, C. L., and T. M. Keller. “Synthesis of Hydroxy-Terminated, Oligomeric Poly(Silarylene Disiloxane)s via Rhodium-Catalyzed Dehydrogenative Coupling and Their Use in the Aminosilane-Disilanol Polymerization Reaction.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 40, no. 9, pp. 1334–1341, 2002.
- Keller, T. M. “Oxidative Protection of Carbon Fibers With Poly(Carborane-Siloxane-Acetylene).” Carbon, vol. 40, no. 3, pp. 225–229, 2002.
- Beckham, H. W., and T. M. Keller. “Diacetylene-Terminated Diacetylene-Containing Polysiloxanes.” Journal of Materials Chemistry, vol. 12, no. 12, pp. 3363–3365, 2002.
- Kolel-Veetil, M. K., and T. M. Keller. “The Effects of Concentration Dilution of Cross-Linkable Diacetylenes on the Plasticity of Poly(m-Carborane-Siloxane-Diacetylene)s.” Journal of Materials Chemistry, vol. 13, no. 7, pp. 1652–1656, 2003.
- Kolel-Veetil, M. K., H. W. Beckham, and T. M. Keller. “Dependence of Thermal Properties on the Copolymer Sequence in Diacetylene-Containing Polycarboranylenesiloxanes.” Chemistry of Materials, vol. 16, no. 16, pp. 3162–3167, 2004.
- Kolel-Veetil, M. K., S. B. Qadri, M. Osofsky, and T. M. Keller. “Formation of a Superconducting Mixture of β-Mo2C Nanoparticles and Carbon Nanotubes in an Amorphous Matrix of Molybdenum Compounds by the Pyrolysis of a Molybdenum Derivative of a Carboranylenesiloxane.” Chemistry of Materials, vol. 17, no. 24, pp. 6101–6107, 2005.
- Kolel-Veetil, M. K., S. B. Qadri, M. Osofsky, T. M. Keller, R. Goswami, and S. A. Wolf. “Size-Induced Effects on the Superconducting Properties of Mo2C Nanoparticles.” Journal of Physical Chemistry C, vol. 111, no. 45, pp. 16878–16882, 2007.
- Anderson, P. W. “Theory of Dirty Superconductors.” Journal of Physics and Chemistry of Solids, vol. 11, nos. 1–2, pp. 26–30, 1959.
- Kolel-Veetil, M. K., S. B. Qadri, M. Osofsky, R. Goswami, and T. M. Keller. “Carbon Nanocapsule-Mediated Formation of Ferromagnetic Fe5Si3 Nanoparticles.” Journal of Physical Chemistry C, vol. 113, no. 33, pp. 14663–14671, 2009.
- Kolel-Veetil, M. K., D. D. Dominguez, C. A. Klug, and T. M. Keller. “Hydrosilated Dendritic Networks of POSS Cores and Diacetylene Linkers.” Macromolecules, vol. 42, no. 12, pp. 3992–4001, 2009.
- Kolel-Veetil, M. K., K. P. Fears, S. B. Qadri, C. A. Klug, and T. M. Keller. “Formation of a Crosslinked POSS Network by an Unusual Hydrosilylation: Thermo-Oxidative Stabilization of the α-Cristobalite Phase in Its Amorphous Regions.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 50, no. 15, pp. 3158–3170, 2012.
- Kolel-Veetil, M. K., D. D. Dominguez, C. A. Klug, K. P. Fears, S. B. Qadri, D. Fragiadakis, and T. M. Keller. “Hybrid Inorganic-Organic Poly(Carborane-Siloxane-Arylacetylene) Structural Isomers With In-Chain Aromatics: Synthesis and Properties.” Journal of Polymer Science, Part A: Polymer Chemistry, vol. 51, no. 12, pp. 2638–2650, 2013.
Biography
Dr. Manoj Kolel-Veetil is a research chemist in the Chemistry Division at NRL, Washington, DC, where he started as an ASEE Postdoctoral Research Fellow and transitioned to federal employment. At NRL, he worked on several areas of chemistry, including hybrid inorganic-organic polymers, cyclic peptide nanotubular materials, boron carbide-based materials, ceramic materials, and the science of per- and polyfluoroalkyl substances. He holds a Ph.D. in chemistry from the University of Vermont and subsequently conducted postdoctoral research at Kansas State University and the University of Oklahoma.