Introduction
Corrosion costs the U.S. Department of Defense (DoD) billions of dollars annually and heavily impacts the availability of DoD assets to carry out their missions [1]. The U.S. Navy is the hardest hit branch of the DoD, as it operates in the harshest environments with its assets exposed to seawater for extensive time. In the 2021 “DoD Corrosion Prevention and Control Strategy” [2], corrosion maintenance and repair were estimated to cost the Navy $7 billion annually and led to 11 million hours of maintainer repair time and lost flight time (as exemplified in Figure 1a). In the time it took to read the previous sentence, corrosion cost the Navy $2,219.68. Not only are ships and submarines constantly in direct contact with seawater, but Naval aircraft also operate primarily in the marine boundary layer containing high levels of aerosolized salts. These sea salt aerosols contribute to aircraft corrosion while on a ship’s flight deck, during normal flight operations, and at land-based Navy installations. Naval Air bases are typically near seawater but can vary from a few miles up to hundreds of miles. Sea-spray aerosols can remain in the air for at least 25 km, contributing heavily to the amount of corrosion observed near coastal areas [3].

Figure 1. (a) Corrective Maintenance of Nonskid Coating on a Ship Deck (Source: U.S. DoD) and (b) Preventative Maintenance via Aircraft Rinsing (Source: U.S. Air Force).
Recently, there have been discussions about moving from a fixed maintenance schedule to one driven by condition-based maintenance (CBM or CBM+). Under this new paradigm, assets would be inspected, washed, and serviced based on the actual condition. Specifically, this type of maintenance strategy would alter wash frequencies of aircraft to higher or lower frequencies, depending on the corrosion risk at the operational site (Figure 1b). One of the simplest ways to do this is to differentiate sites by their proximity to a saltwater source. However, this may not always be the best practice. Ongoing work by DoD partners is examining this assumption by categorizing corrosion risk on a smaller scale and at individual locations rather than only using a map. The local environment plays a large role in salt deposition, from wind direction and intensity to local vegetation, and moving just half a mile away can sometimes reduce the corrosion risk by an order of magnitude. It is imperative that these local impacts are considered when categorizing a site and determining maintenance intervals.
Corrosion Severity in the Environment
Corrosion processes are affected by environmental conditions, which can change the rate, chemistry, and morphology of the corrosion attack [4, 5]. Many climate types have been categorized in corrosion literature—rural, suburban, urban, forest, highway, coastal/marine, industrial, alpine, tropical, volcanic, agricultural, and dry [6–8]. These sites are characterized by weather parameters and the chemistry of the atmosphere, which result in differences in corrosion across the different climate types, with marine and industrial sites often showing the highest levels of corrosion. Commonly measured weather parameters in corrosion studies include temperature, relative humidity, precipitation, solar radiation, and wind speed and direction. Because corrosion is an electrochemical process, a conductive moisture layer (very thin at times) is required on a metallic surface for corrosion reactions to occur, and the presence and characteristics of this layer are affected by these meteorological factors [9]. Thus, environments that are very hot and dry (which prevent the formation of these moisture layers on samples) tend to show low corrosion rates [4, 5].
The atmospheric chemistry also plays a role in the corrosion processes. One of the most important environmental species is chloride ion, which is a major driver of the high corrosivity seen in many coastal and marine environments [7]. Other industrial pollutants such as sulfur and nitrogen oxide species and the ozone have also been shown to accelerate corrosion [10]. The proximity to the source of these corrosion-accelerating compounds will determine their concentration and effect at any given site. Other parameters like wind strength and direction, surrounding vegetation, and other structures will also play a role.
An interesting example of the effect of climate on corrosion processes is a study that was done in Hawaii [3, 8]. Across the main Hawaiian island and the island of Oahu, eight different corrosion test sites were chosen to characterize the corrosion severity of the seven climatological environments across the state—marine, industrial, tropical, volcanic, alpine, agricultural, and dry. A wide range of environmental conditions (temperature, humidity, rainfall, solar radiation, chloride ion deposition rates, and other environmental chemistry factors) was observed across the test sites. These environmental differences resulted in corrosion rates that varied by up to a factor of 40.
Accelerated Corrosion Testing and Modeling
Although there are American Society for Testing and Materials (ASTM) test methods for exposing bare and coated metals in a controlled, corrosive environment to determine relative corrosion resistance and coating behavior, the prediction and correlation of corrosion performance of “accelerated exposure tests” in environmental chambers like salt spray (B117) to field environments are not always straightforward [11]. Existing test methods do not address the simultaneous exposure of various atmospheric and environmental conditions that can affect corrosion performance of a bare or coated metal. They also do not address the need to correlate results of exposure chamber tests with exposure to outdoor atmospheres and end-user performance. Many investigations have been performed in recent years to clarify the role of environmental and climatic factors in the atmospheric corrosion of commonly used structural metals and coatings as well as simulate their observed corrosion behavior in the laboratory [12–16]. It is well documented that corrosion behavior of metal substrates in accelerated laboratory tests does not correlate with the observed performance in an outdoor exposure environment [17–19].
The first step toward developing better accelerated test methods is to analyze and accurately reproduce these environments in a laboratory setting. A successful, accelerated test method would therefore be environmentally “tunable” and provide accurate, predictable results for any substrate with any type of protective barrier layer or coating present. To achieve this goal, a new state-of-the-art accelerated combined effects simulation (ACES) exposure chamber is currently undergoing testing by the U.S. Air Force (USAF) Research Laboratory in collaboration with the U.S. Naval Research Laboratory (NRL) to replicate field conditions and corrosion behavior of various alloys, coating systems, and corrosion/environmental sensors. This chamber was built to include more environmental effects than any previous exposure chamber to accurately mimic a field environment that includes temperature and humidity control, ultraviolet (UV), mixed gasses, and actuators to simulate mechanical stresses.
Background of Environmental Severity Indices
Early attempts to correlate the environment with corrosion of DoD assets goes as far back as the 1960s under the U.S. Air Force Logistics Command, where the focus was to develop a corrosion severity classification for each operational airbase as part of the Corrosion Prevention and Control program (redesignated as Project RIVET BRIGHT in 1971) [20]. The program was redesignated as PACER LIME in 1972 and was a two-phase effort—develop a mathematical algorithm to calculate a corrosion factor that combined weather and other environmental factors and measure corrosion severity at selected locations through atmospheric tests, allowing for the calibration of the corrosion factor calculated from the algorithm. This corrosion factor had a range of values: 1.00–2.00 (severe), 2.01–2.85 (moderate), and 2.85–3.75 (mild). As a result of that study, a system was developed for rating the corrosivity of aircraft operational environments, considering environmental variables such as weather, atmospheric pollutants, and geographical factors by Summitt and Fink [20]. The purpose was to compute a corrosion severity index for three aspects of corrosion maintenance—aircraft washing, repainting, and maintenance repairs. These indices were derived from the corrosion factor ranges and were thus labeled as mild, moderate, and severe. The corrosion severity index for each airbase location was then used to schedule the frequency of aircraft wash cycles. It was reported that the computed severity ratings agreed with aircraft maintainers’ experience and atmospheric testing programs at several DoD locations. These indices were incorporated into Technical Order (TO) 1-1-691 in 1996 [21].
Meanwhile, the Naval Air Systems Command (NAVAIR) 01-1A-509 [22] and 16-1-540 [23] Technical Manuals (TMs) were combined in 2005 into NAVAIR TMs 01-1A-509 Volumes I (Corrosion Program and Corrosion Theory) [24], Volume II (Aircraft) [25], and Volume III (Avionics and Electronics) [26], which superseded NAVAIR TO 1-1-689 [27] from 2000.
In 2008, Battelle Columbus published “A Decade of Corrosion Monitoring in the World’s Military Operating Environments: A Summary of Results” [28]. It describes an effort (1998–2008) to quantify and refine categories in the USAF TO 1-1-691 [21] in 2009 for aircraft wash cycles and maintenance actions. This attempt was based upon Battelle’s program of placing “corrosion monitoring packages” consisting of well-defined corrosion cards containing bare metal coupons (i.e., similar sized, cleaned, and initial mass values recorded) at military bases worldwide for predetermined periods. Upon return from the field, the coupons were cleaned and the resulting mass loss per unit area for each coupon was measured and recorded, thus yielding a corrosion rate for the exposure period at a particular site.
One of the results of this study was an algorithm for corrosion prediction based upon the corrosion card mass loss and silver chloride film measurements, which only considered environments at greater than 70% relative humidity (RH), excluding the effect of temperature and sulfur dioxide in the statistical analysis [28]. The resulting Environmental Severity Index (ESI) classifications were based upon the corrosion rate of AA2024-T3 aluminum and also described as mild, moderate, or severe. International Organization for Standardization (ISO) Standard 9223:2012(E) [29] lists six different corrosivity categories (low to extreme) and uses the percentage exposure time above 80% RH. Overall, more than 40 years of modeling attempts have been made to predict the rate of atmospheric corrosion and/or severity for a given metal substrate [30]. While numerous models have been developed, they all require inputs of environmental data as well as corrosion mass change data; obviously, a model is only as good as the data used for its calibration and development. It is apparent that very few, if any, of the models developed were ever independently validated [30].
Recent Navy Efforts
Strategic Environmental Research and Development Program – Environmental Security Technology Certification Program (SERDP-ESTCP) Projects
Current work funded by SERDP-ESTCP is examining the link between corrosion risk and environmental implications. The environmental and mechanical loading conditions govern the overall lifetime survivability and maintenance cycles of protective coatings. A model that can better predict maintenance based on accumulated damage will enable maintenance cycles to be performed only when necessary, as opposed to overly conservative, periodic time-based maintenance intervals based on worst-case scenarios. Using a CBM+ approach reduces the exposure of both personnel and the environment to hazardous paint strippers and hexavalent chromium used in many primers. By evaluating each asset based on service history and expected future exposure, maintenance will be done when required based on usage/exposure history. To achieve the desired state, better modeling of coating and material lifetime performance need to be developed.
Furthermore, a combined model of real-world fatigue and corrosion damage for DoD assets does not exist. By generating data that simulates a real-world environment and inputting this information into a predictive model, better decisions can be made to the service intervals and reducing the number of man-hours spent on systems with potentially hazardous materials to refurbish the asset. The objective of this project is to generate a Bayesian network model to predict coating and lifetime performance based on a CBM+ approach. The resulting model could be used to better inform life-cycle maintenance costs and adjust the schedules for planned maintenance. Successful implementation will reduce inspection costs and unnecessary repainting and better prioritize key asset maintenance. Model inputs should reflect what the real-world experience of the asset is in terms of environment, loading, and maintenance. Figure 2 shows what some of the considerations are for studying DoD aircraft (Figure 2a). Tailored for tackling DoD needs, the NRL-Key West facility implements rinsing and covering modifications (Figure 2b) to mimic what aircraft experience. Fasteners (Figure 2c) are places where protective coatings are often breached and material incompatibility leads to corrosive attack, so modeling efforts are concentrated at these hot spots. Dynamic loading of simulated aircraft structures (panels, fasteners, coatings, etc.) in outdoor environments provides a higher level of testing fidelity (Figure 2d).
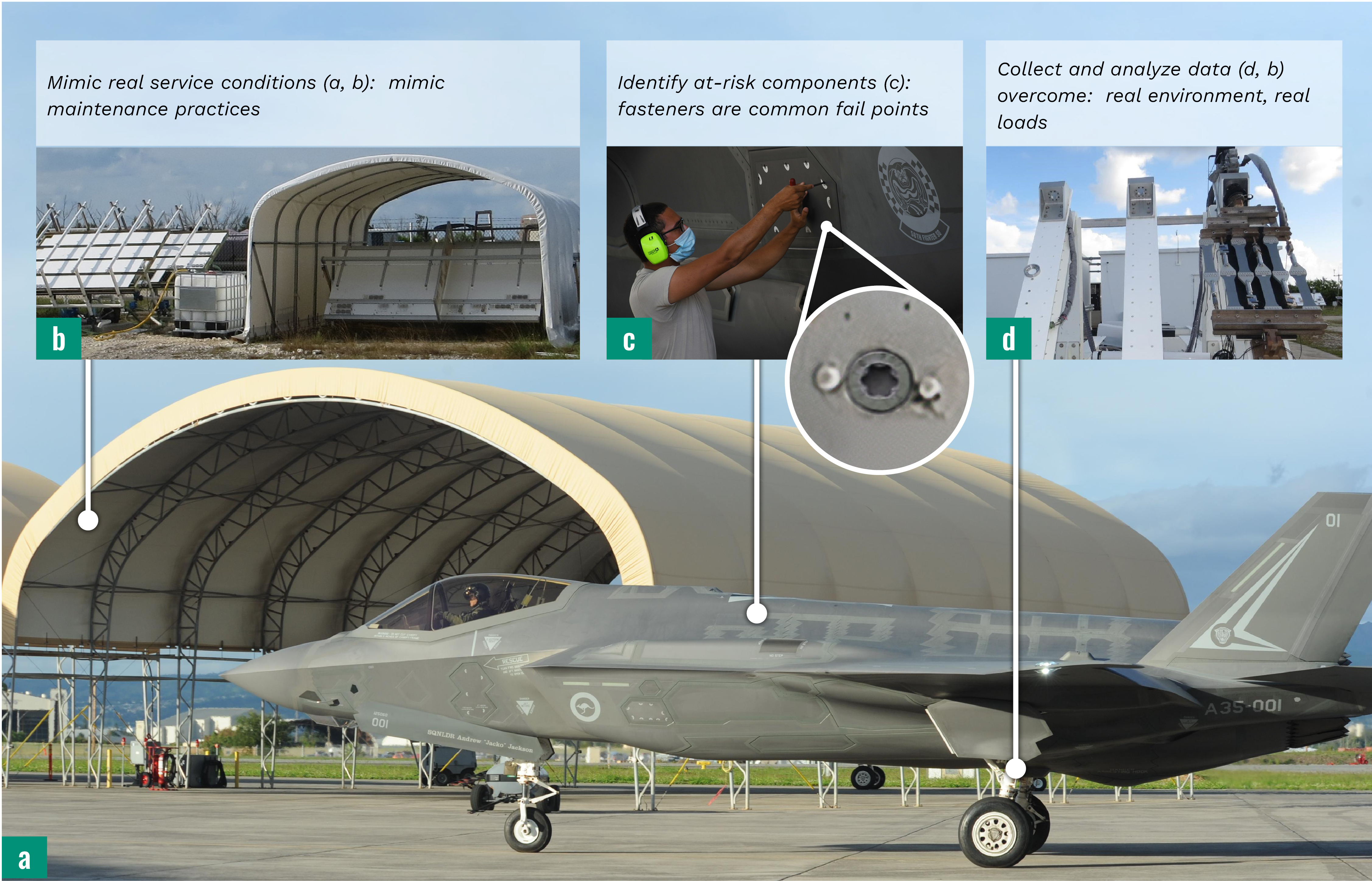
Figure 2. One Strategic Outlook on How the DoD Invests Research Into Atmospheric Corrosion to Counteract Its Effect on Mission Readiness (Sources for a and c Are U.S. Air Force; b and d Are S. Stanke, Excet).
Field Exposure Testing
Current efforts to improve ESI research and development include standardizing how ESI work is conducted. The Association for Materials Protection and Performance (AMPP) SC-07 Ad Hoc Group is developing a standard titled “Environmental Spectra for Severity Classification,” which brings together the atmospheric corrosion community to codify best practices and state-of-the-art understanding of the current problem. Three focus areas include sample design (what samples will be exposed and how), data acquisition (how data are collected), and data analysis (how data are interpreted and what the significance is for ESI). Critically, this standard will consolidate a common vocabulary across the field, allow data sharing and encourage collaborations, enable a data network for long-term and large-area assessments, increase measurement accuracy and precision, and provide well-defined inputs for modeling efforts. Examples of what this standard addresses based on currently agreed-upon best practices include sample size and classification, data acquisition through coulometric reduction and surface profilometry, and core environmental variables to collect during exposure. The samples on the rack in Figure 3 show sample size and classification, and the large vertical box behind the rack collects the core environmental variables.

Figure 3. Instrumented Exposure Rack With Corrosion Test Coupons at NRL-Key West (Source: D. Hansen).
Corrosion Modeling
Several DoD-funded corrosion modeling efforts are currently underway that involve various forms of data collected from different locations. Atmospheric corrosion proceeds via several processes in sequence and/or parallel across multiples classes of matter—the atmosphere, condensed aqueous solution, polymer coatings, oxide scales, precipitated salts, and microstructurally heterogeneous metal alloys (see Figure 4). Multiple physical and chemical phenomena contribute to the corrosion process, including mass transport, electrochemical effects, metal dissolution, grain-boundary transport, etc. For this reason, using fundamental physics or chemical principles makes it difficult to directly predict the corrosion rate of a metal in its environment. Likewise, it is difficult to directly extrapolate the results of short-term tests to long-term tests solely from physical principles. A data-driven modeling approach can assist in identifying the key environmental factors driving atmospheric corrosion.
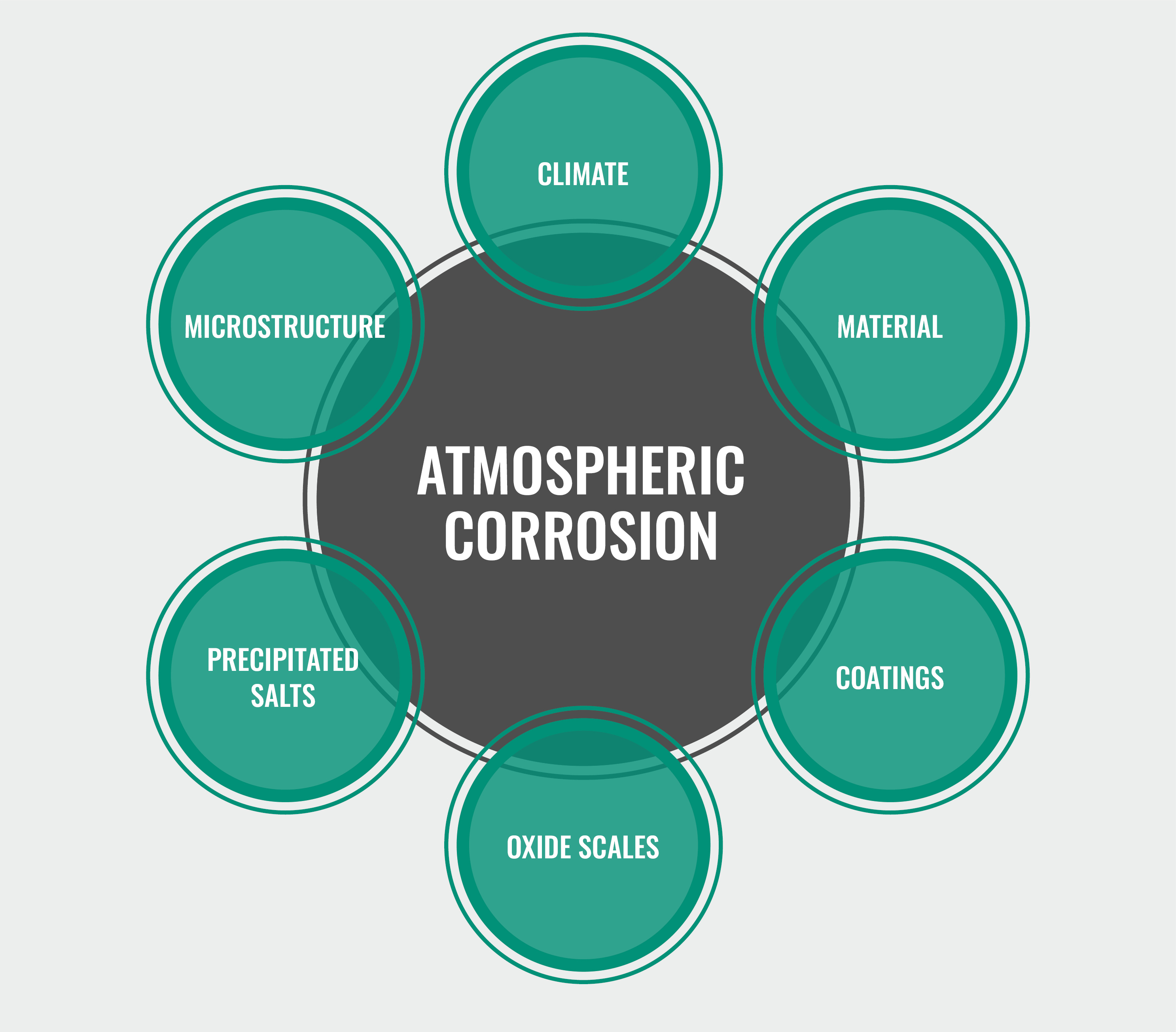
Figure 4. Components Needing Consideration in Modeling of Atmospheric Corrosion—Types of Material, Surface Types, Deposits, and Time-Dependent Processes [24].
One example is the use of machine learning (ML) and artificial neural network (ANN) modeling to identify leading meteorological factors that quantitatively control the extent of corrosion [24]. The systematic collection of atmospheric corrosion data has enabled the application of ML techniques to understand the most critical elements (Figure 4) impacting the differences in the amounts of corrosion observed in AA2024-T3 samples placed at three locations on the Florida coastline. The data were processed according to the three metrics of mass loss per unit area, linear corrosion rate, and a parabolic corrosion constant, as well as generating additional data by sample differencing that considers cumulative corrosion occurring between time periods. An automated approach was developed that can query public and/or pay-to-access websites for environmental data to construct an exposure profile for a sample placed at a known location (specified by latitude/longitude) and over a given range of dates. ML algorithms (feature selection and ANNs) were used to determine the most significant environmental features impacting the extent of atmospheric corrosion through sensitivity analysis. Five key variables were determined to have a quantitative effect on the corrosion rate and mass loss per unit area collected over 18 months of exposure—mean precipitation, the range of temperatures, the minimum wind speed, the variability of ozone exposure, and the maximum solar irradiance [31]. This systematic approach could be applied to other materials of interest, different locations, and other metrics of corrosion (e.g., localized corrosion depth and pitting volume) to advance the understanding of how the environmental conditions can directly influence the corrosion behavior of materials.
Challenges Ahead
Transitioning from a schedule-based maintenance program to a condition-based maintenance program within the DoD requires several issues to consider—how to categorize the corrosion severity occurring on the assets in the field and how to use the classifications to make informed decisions relating to weapon system maintenance, as well as DoD infrastructure (construction, storage, etc.). Another issue is how many classifications are appropriate. Are three categories enough (TO 1-1-691 as mild, moderate, or severe), six (ISO 9223 as C1-CX), or possibly 10? Studies have shown that it is possible to get significantly different levels of corrosion for different materials; so which should be used for the severity ranking? If the goal is to determine a corrosion severity for each DoD location, is it possible to rank a facility with more than one category, or do we use an average for multiple locations at the facility? Ultimately, can corrosion and environmental sensors reliably provide real-time information so that the corrosion severity of a location can be determined, allowing maintenance cycles to be adapted for season-season or year-year variability or even longer-term effects like climate change?
Conclusions
Collaborative efforts continue among the research arms of the Navy and Air Force to standardize the field exposure tests yielding data that will be used to categorize the corrosion severity rankings for DoD locations. In addition to field exposure testing, a new state-of-the-art ACES exposure chamber is currently undergoing testing to replicate field conditions and corrosion behavior of various alloys, coating systems, and corrosion/environmental sensors. The combination of real-world corrosion data from depot and field exposure test sites, advanced modeling techniques utilizing artificial intelligence and ML, and cutting-edge exposure simulation chambers will ultimately provide a basis for a more accurate characterization of the corrosion severity for DoD locations around the world. Realization of a condition-based maintenance program across the services is not as far away as once thought. 
References
- Herzberg, E. F., T. K. Chan, S. Guo, A. K. Morris, A. Stevenson, and R. F. Stroh. “Estimated Impact of Corrosion on Cost and Availability of DoD Weapon Systems – FY18 Update.” LMI, Tysons, VA, 2018.
- U.S. DoD. “DoD Corrosion Prevention and Control Strategy.” Office of the Under Secretary of Defense for Acquisition and Sustainment, Washington, DC, 2021.
- de Leeum, G., F. P. Neele, M. H. Smith, and E. Vignati. “Production of Sea Spray Aerosol in the Surf Zone.” Journal of Geophysical Research, vol. 105, no. D24, pp. 29397–29409, 2000.
- Yoon, Y., J. D. Angel, and D. C. Hansen. “Atmospheric Corrosion of Silver in Outdoor Environments and Modified Accelerated Corrosion Chambers.” Corrosion, vol. 72, no. 11, pp. 1424–1432, 2016.
- Petry, L., and D. C. Hansen. “A Comparative Study of Two Coating Systems Exposed for Two Years in the Field and in Accelerated Atmospheric Corrosion Chamber Environments.” Corrosion, vol. 72, no. 11, pp. 1385–1395, 2016.
- Oesch, S., and P. Heimgartner. “Environmental Effects on Metallic Materials – Results of an Outdoor Exposure Programme Running in Switzerland.” Materials and Corrosion, vol. 47, pp. 425–438, 1996.
- Sun, S., Q. Zheng, D. Li, and J. Wen. “Long-Term Atmospheric Corrosion Behaviour of Aluminum Alloys 2024 and 7075 in Urban, Coastal, and Industrial Environments.” Corrosion Science, vol. 51, pp. 719–727, 2009.
- Srinivasan, R., and L. H. Hihara. “Correlation Studies Between Outdoor Exposure and Accelerated Laboratory Corrosion Tests for Galvanic and Non-Galvanic Ceramic-Aluminum Couples.” ECS Transactions, vol. 16, pp. 101–113, 2009.
- Saberi, L., and M. Amiri. “Modeling Atmospheric Corrosion Under Dynamic Thin Film Electrolyte.” Journal of The Electrochemical Society, vol. 168, p. 081506, 2021.
- Oesch, S., and M. Faller. “Environmental Effects on Materials: The Effect of the Air Pollutants SO2, NO2, NO, and O3 on the Corrosion of Copper, Zinc, and Aluminum. A Short Literature Survey and Results of Laboratory Exposures.” Corrosion Science, vol. 39, no. 9, pp. 1505–1530, 1997.
- ASTM International. “B117 Standard Practice for Operating Salt Spray (Fog) Apparatus.” West Conshohocken, PA, 2019.
- Chen, Z., D. Liang, G. Ma, G. Frankel, H. Allen, and R. Kelly. “Influence of UV Irradiation and Ozone on Atmospheric Corrosion of Bare Silver.” Corrosion Engineering Science and Technology, vol. 45, pp. 2169–2180, 2010.
- Wan, Y., E. Macha, and R. Kelly. “Modification of ASTM B117 Salt Spray Corrosion Test and Its Correlation to Field Measurements of Silver Corrosion.” Corrosion, vol. 68, no. 3, pp. 036001-1–036001-10, 2012.
- Feng, Z., G. Frankel, W. Abbott, and C. Matzdorf. “Galvanic Attack of Coated Al Alloy Panels in Laboratory and Field Exposure.” Corrosion, vol. 72, no. 3, pp. 342–355, 2016.
- Tayler, M., M. Blanton, C. Konecki, J. Rawlins, and J. Scully. “Scribe Creep and Underpaint Corrosion on Ultra-High Molecular Weight Epoxy Resin Coated 1018 Steel Part 1.” Corrosion, vol. 71, no. 1, pp. 71–91, 2015.
- Tayler, M., M. Blanton, C. Konecki, J. Rawlins, and J. Scully. “Scribe Creep and Underpaint Corrosion on Ultra-High Molecular Weight Epoxy Resin Coated 1018 Steel Part 2.” Corrosion, vol. 71, no. 3, pp. 326–342, 2014.
- King, A., B. Kannan, and J. Scully. “Environmental Degradation of a Mg Rich Primer in Selected Field and Laboratory Environments: Part 1 – Without a Topcoat.” Corrosion, vol. 70, no. 5, pp. 512–535, 2014.
- Liang, D., H. C. Allen, G. S. Frankel, Z. Y. Chen, R. G. Kelly, Y. Wu, and B. E. Wyslouzil. “Effects of Sodium Chloride Particles, Ozone, UV and Relative Humidity on Atmospheric Corrosion of Silver.” Journal of the Electrochemical Society, vol. 157, no. 4, pp. C146–C156, 2010.
- Deflorian, F., S. Rossi, L. Fedrizzi, and C. Zanella. “Comparison of Organic Coating Accelerated Tests and Natural Weathering Considering Meteorological Data.” Progress in Organic Coatings, vol. 59, no. 3, pp. 244–250, 2007.
- Summitt, R., and F. T. Fink. “AFWAL-TR-80-4102 PACER LIME: Part I – An Environmental Corrosion Severity Classification System.” USAF, Dayton, OH, 1980.
- USAF TO 1-1-691. “Cleaning and Corrosion Prevention and Control, Aerospace and Non-Aerospace Equipment.” Dayton, OH, 1996.
- NAVAIR TM 01-1A-509. “Avionics Cleaning and Corrosion Prevention/Control.” Patuxent River, MD: Naval Air Systems Command, 2001.
- NAVAIR TM 16-1-540. “Avionics Cleaning and Corrosion Prevention/Control.” Patuxent River, MD: Naval Air Systems Command, 2000.
- NAVAIR TM 01-1A-509-1. “Cleaning and Corrosion Control Volume I Corrosion Program and Corrosion Theory.” Patuxent River, MD: Naval Air Systems Command, 2005.
- NAVAIR TM 01-A-1A-509-2. “Cleaning and Corrosion Control Volume II Aircraft.” Patuxent River, MD: Naval Air Systems Command, 2005.
- NAVAIR TM 01-1A-509-3. “Cleaning and Corrosion Control Volume III Avionics and Electronics.” Patuxent River, MD: Naval Air Systems Command, 2005.
- NAVAIR TM 1-1-689. “Avionics Cleaning and Corrosion Prevention/Control.” Patuxent River, MD: Naval Air Systems Command, 2000.
- Abbott, W. “A Decade of Corrosion Monitoring in the World’s Military Operating Environments: A Summary of Results.” Battelle Memorial Institute, Columbus, OH, 2008.
- ISO Standard 9223:2012(E). “Corrosion of Metals and Alloys – Corrosivity of Atmospheres – Classification, Determination and Estimation,” 2012.
- Rose, D. H. “A Cumulative Damage Approach to Modeling Atmospheric Corrosion of Steel.” Dayton, OH: University of Dayton, 2014.
- Taylor, C. D., D. Borth, and D. C. Hansen. “Environmental Exposure Profiles for Atmospheric Corrosion.” In AMPP Annual Conference + Expo, Denver, CO, 2023.
Biographies
Douglas C. Hansen is a distinguished research scientist at the University of Dayton Research Institute (UDRI) and professor (joint appointment) in the Department of Chemical and Materials Engineering in the School of Engineering at the University of Dayton. He has more than 30 years of experience in protein biochemistry and corrosion electrochemistry, primarily in the development of environmentally friendly coatings and corrosion inhibitors. Dr. Hansen holds a Ph.D. in marine biochemistry from the University of Delaware studying marine biopolymers as corrosion inhibitors.
Christine E. Sanders is the lead principal investigator for airframe corrosion efforts at NRL. Her current research includes atmospheric corrosion mechanisms and modeling, environmental exposure to simulate operational conditions, and maintenance cycle optimization. Dr. Sanders holds a Ph.D. in physical chemistry from The Ohio State University studying the atmospheric corrosion processes of silver.
Ronald A. Zeszut is a senior researcher for UDRI. His research includes atmospheric corrosion, silver corrosion, nondestructive surface characterization techniques, biomaterials, and engine lubricants. Dr. Zeszut holds a Ph.D. in chemical engineering from Case Western Reserve University studying electroless deposition of copper for semiconductor applications.
Raymond J. Santucci is a materials engineer conducting research on atmospheric corrosion for the DoD at NRL. His research includes environmental severity prediction and monitoring techniques, machine learning, and additive manufacturing. Dr. Santucci holds a Ph.D. from the University of Virginia upon completing his research on sacrificial aerospace coatings.
Matthew J. Rothgeb is a principal research scientist and group leader at UDRI. He manages the Coatings, Corrosion, and Erosion Laboratory at Wright-Patterson Air Force Base. His research includes studying the corrosive effects of multivariate environmental exposures on metals and corrosion preventative materials, coatings development, testing, and subsequent qualification of coatings to international and military specifications. Mr. Rothgeb holds an M.S. in engineering management sciences from the University of Dayton.


