With recent advances in medical technology and combat casualty care, today’s wounded warrior (such as the one discussed in the companion article, “An Account of a Future Wounded Warrior,” on page 5) is being supported with state-of-the-art treatment materials and techniques never before seen. As a result, critical injuries can be repaired more rapidly; nonrecoverable limbs can be replaced more effectively; and lives can be saved more often. In this article, we examine some of the major technologies that are expected to help make these improved outcomes possible.
MEDICAL MATERIALS AND WOUND CARE
Simply put, the battlefield is an extremely dangerous place. Many sharp edges moving at high velocity and expansive projectiles moving at even higher velocities represent a tremendous danger to the Warfighter. Likewise, concussive waves and effects from exploded ordnance can significantly minimize or negate the protection afforded by the use of body armor. Traditionally, a Warfighter coming in contact with these combat threats has meant that, at best, medical teams were likely going to have their hands full and, at worst, there was a strong possibility of losing valued combat personnel.
Fortunately, solutions are becoming available to counteract these injury scenarios to the Warfighter, both in the near term and future. In the areas of both internal and external hemorrhaging, several unique materials and techniques are emerging to help sustain a Warfighter against his/her wounds. As discussed in the sections that follow, these include expansive medical foams, doped primary care (DPC) wound dressings, and hydrogel scaffolds.
Expansive Medical Foams
Consider the age-old elementary school science project: the volcano. When certain measures of vinegar and baking soda are combined, the material expands for a brief period of time as gas and bubbles. Via a similar principle, foam is simply that reaction with a suspender added so as to retain structure. Similarly, consider the behavior of a two-part epoxy system, which is typically used in adhesion processes. Two separate materials, when combined, produce a new material that solidifies. When combining the properties of foam generation, two-part epoxy systems, and biomedical application, the result is expansive medical foam (as shown in Figure 1). These foams are intended to be hemostatic in nature, meaning that their purpose is to stop the bleeding as quickly as possible. Their component structures must therefore be biocompatible and reduce the lethality of a severe internal bleed.
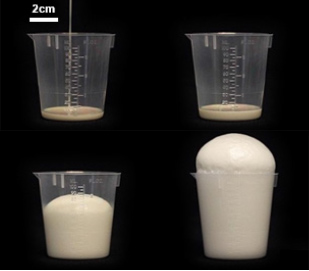
Figure 1: Example Foam Volume Expansion.
Through a Defense Advanced Research Projects Agency (DARPA) grant, the Massachusetts-based company Arsenal Medical has developed and tested an internal hemostatic foam compound based on polyol and isocyanate (common materials in electrospinning processes). The foam is injected percutaneously as a two-part mixture to create, within the abdominal cavity, a self-expanding hemostatic polyurethane foam for far-forward hemorrhage control.
In massive noncompressive bleeding testing, the results of this applied foam increased the 3-hr survival percentage nearly tenfold. Likewise, the mean survival time was increased from less than 1 hr with high variability to more than 3 hr with a smaller variability. This result means that sustained internal bleeding could be kept at bay for more than 3 hr before a surgical team is able to repair the lacerations causing the bleed. Accordingly, expansive foam is a solution that could have life-or-death implications on the battlefield.
While larger companies such as Johnson-Johnson and 3M have also begun to explore the possibility of hemostatic foams, practically no competition exists on a research basis for the work that Arsenal has successfully demonstrated. Accordingly, the company recently debuted a spinoff company, 480 Biomedical, to focus exclusively on the development of hydrogel scaffolds for the treatment of occluding diseases in the femoral artery.
DPC Dressings and Hydrogel Scaffolds
DPC wound dressings are essentially specialized fiber dressings embedded at the nano-scale with chemicals to assist with assorted mechanisms of drug delivery. Applying such a bandage to a surface wound on a Warfighter can have multiple effects: it can draw out bacteria from a wound, act as a hemostatic agent (with no pressure required) (as illustrated in Figure 2), and deliver critical drugs transdermally without the presence of a wound. These unique effects and mechanisms are generated from fibers that are electrospun.
Electrospinning a fiber is the mechanism by which a polymer-laden fluid can be electrically excited into depositing itself in an extremely thin stream down to a ground plate. Upon reaching the plate, the fiber’s solvent will have evaporated, and what is left is a woven fabric imbued with chemical structures within the fibers at the nano-scale. The surface-to-volume ratio on such fibers is absolutely unmatched anywhere in metallurgical or other material pursuits, allowing for highly unique effects on human tissue interaction.

Figure 2: DPC Bandage Clotting Blood Through Ionic Interaction.
As illustrated in Figure 3, a basic electrospin system consists of an electrical power supply, a needle syringe, a ground plate, and a pump of some kind. A high voltage potential (on the order of several kilovolts) is applied to the needle syringe, and the plate below the syringe is grounded in a preconceived manner. As solution is pumped into the syringe, the voltage potential separating the air gap between the syringe tip and ground causes a droplet to gradually lose its surface tension. As a result of this loss, an extremely thin stream of solution will begin to flow from this droplet, called a Taylor Cone, down to the base plate. The manner in which this solution meets the ground contacts can be controlled by a computer, with several grounds being connected into the circuit at different locations at different times. This will control the weave of the electrospun fiber.
The contents of solution that are deposited onto the ground plate are myriad in variety; however, developing new solutions also brings with it some characterization problems. Finding the correct voltage and Taylor Cone geometry requires extensive in-lab testing, usually on a trial-and-error basis. Research has led to several well-observed solutions and solvents that behave predictably in the high-voltage potential environment and that are thus prime candidates for DPC wound dressings
Most promising on this front are chitosan-doped fibers. Chitosan is a peculiar element in that is derived from an unlikely source: crustaceans. When shrimp/crab shells are mixed with sodium hydroxide (in excess with water as a solvent), the resulting product is chitosan. Two of the most useful properties of chitosan are its ability to act as a hemostatic agent and as an antibacterial compound. Chemically, what also makes chitosan attractive as a delivery agent is that, after interaction with the sodium hydroxide, its polymeric structure becomes protonated. This quality delivers the advantage of water solubility and strong binding to negatively charge surfaces (e.g., mucosal membranes). Thus, chitosan simultaneously functions as a highly efficient hemostatic agent, antibacterial, and drug delivery system [1].
That said, chitosan-doped fibers on their own are not quite enough to provide an advanced wound care package. Difficulties arise in scaling up the thickness of electrospun chitosan-doped fibers due to the electrospin process itself. At approximately 200 μm (in the vertical direction) from the ground plate, fiber flow becomes unpredictable and no longer adheres to itself. It is believed through observation that this negative side effect is caused by the distance from the ground plate. To counteract this effect, a hydrogel (scaffolding agent) can be used to separate layers and continue the thickness growth process.
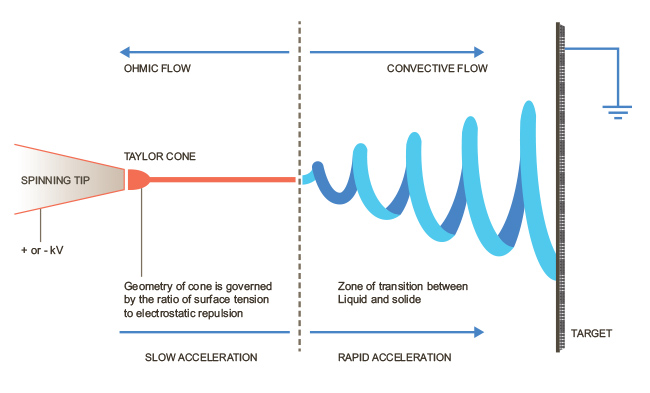
Figure 3: Electrospin Process.
This extensional growth process begins to cross over into the territory of tissue engineering, as the scaffolding principles with hydrogels are the same [1]. However, in lieu of printing cellular material, the doped fibers of the electrospin process are captured within the hydrogel scaffolding (as shown in Figures 4 and 5). This technique’s yield can then be applied as a wound dressing. Now there exists DPC wound dressing capable of stopping traumatic blood flow, disinfection, and drug delivery simultaneously.
Unfortunately, electrospinning is not yet performed outside of research labs. While basic electrospin equipment is not inherently expensive, the parameters to generate predictable fiber behavior at an industrial level have not been realized. Research groups are currently investing in methods and equipment to scale up electrospun fiber packages using such items as a barrel mandrel to create a wave in the solvent (multiple Taylor Cones for drawing), parallel needle assemblies with multiple ground plates on the same platform, and hybridized hydrogel-chitosan fiber scaffolding techniques borrowed from tissue engineering.
Much of the research efforts developed in the electrospun fiber regime are typically conducted by universities or military labs. Consequently, it can be interpreted that DPC dressings are not yet ready to be integrated into the military supply chain on a large scale. The 3M company produces a hybrid electrospun fabric tradenamed Tegaderm, along with a line of other wound care products made from various materials, such as acrylic, alginate, hydrocolloid, hydrogel, and silver colloids.
PORTABLE STERILIZATION
When discussing sterilization of medical devices, there are two parameters that are of utmost importance: decimal reduction time (D-value) and the sterility assurance level (SAL). The former is an indication of the degree of sterilization expressed by multiples of the D-value, denoting the time needed to reduce the initial number of microorganisms to 1/10th of their original values. It is a common marker in microbiology for indicating effectiveness of an antibacterial method. The SAL value represents the probability of a single unit being nonsterile after it has been subjected to some sterilization process. Typically, the SAL value is represented as 10-X, where X is some integer magnitude to express the probability. The value is verbalized as “log-reduction of” X, implying that the method has been successful at reducing the nonsterilization probability to one Xth of its original value.
With that in mind, we can begin to quantify the methods of sterilization, the most common of which apply the following modalities: heat, chemical, radiation, filtration, pressure, and sonication. As there is interest in the portable aspect of sterilization, a few of these methods may be disregarded. Heat, pressure, and radiation require a significant amount of power and safety equipment to ensure safe operation around humans. Thus, their size is the limiting factor; they are too large for portability. Filtration would be functional for gas sterilization, but medical equipment features are solid in nature. This thus leaves sonication and chemical treatment as the most viable pathways toward a portable sterilization solution.
Sonication Sterilization
Sonication, or ultrasonic cleaning, uses cavitation bubbles induced by high-frequency pressure waves to agitate a liquid. This agitation process produces, via an ultrasonic transducer, compression waves that “tear” the liquid apart, leaving behind cavitation bubbles at the microscopic level. These cavitation bubbles collapse in the wake of the compression wave with tremendous energy; average temperatures and pressures around these bubbles are on the order of 8,500 °F and 20,000 psi, respectively [2]. Unfortunately, the bubbles are so small, they have only the ability to clean and remove surface contaminants. Likewise, the higher the frequency of oscillation, the smaller the cavitation bubble diameters will be. This fact is key to establishing a regime by which ultrasonic waves can be used to sterilize surgical tools. Materials that reduce surface tension, also known as surfactants, greatly enhance the effectiveness of the cavitation phenomena at work in the system [3]. Typically, the apparatus used to ultrasonically clean an object consists of a vessel filled with a surfactant agent along with the objects to be sterilized. A transducer is either lowered into or attached onto the vessel to agitate the fluid medium.
Unfortunately, due to the high SAL values imposed for sterilization, sonication at atmospheric pressure alone is not enough to destroy all biological agents present on an object. However, it has been shown that increasing the pressure from 1 to 2 atm destroyed 90% of bacterial agents present on the work piece. This result represents 1 D-value factor. To meet sterilization standards, 5 more D-value factors must be reached. Thus, it is a step in the right direction. A research team at the Georgia Institute of Technology claims that by circulating the fluid at 2 atm of pressure, it can achieve total sterilization in 10 minutes. If this claim is confirmed, these results would mean it would take 70% less time to sterilize than the current leading low-temperature sterilization method: peracetic acid washing.
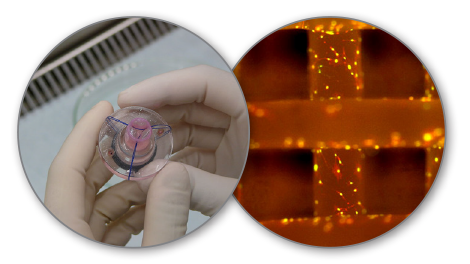
Figure 4 (right): Electrospun Hydrogel/Tissue Scaffold. Figure 5 (left): Tissue Engineered Heart Valve.
Chemical Treatment Sterilization
Chemicals can likewise be used for sterilization. Chemical means are typically chosen if materials have heat sensitivity, such as biologics or electronics. In these instances, either liquid or gas may be used, with each having its own trade-offs. While using chemicals does avoid the problem of heat in the sterilization process, special care must be taken toward workplace safety, as these chemicals are often toxic, are incompatible with some materials, and can catalyze in air (auto combustion).
There are two primary chemicals in use on an industrial level that have varying degrees of success in terms of viability from a portable perspective. These two chemicals, which are discussed in succeeding text, are ethylene oxide (EO) and nitrogen dioxide (NO2). Portability should likewise be defined on a measure typical to armed forces: the highest weight that can be carried by two people, self-contained, minimal power draw, etc.
EO
EO is the most commonly used gas and third most commonly used method for sterilization (behind moist heat and irradiation) [4]. The gas is likewise a raw material with diverse applications in the plastics and food industries. However, EO by itself is a highly hazardous substance. At room temperature, it is an anesthetic gas and is flammable, carcinogenic, mutagenic, irritating, and carries with it a misleading sweet aroma. Likewise, a major safety issue with EO is the fact that its odor threshold is 500 ppm, with a National Institute of Health and Safety “immediately dangerous to life and health” value at 800 ppm. This is an extremely tight range, in addition to the fact that the U.S. Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit to 5 ppm.
As a poison gas that leaves no residue on the items it contacts, pure EO is a disinfectant widely used in hospitals and the medical device industry to replace steam in sterilization of heat-sensitive tools and equipment. And it kills everything: spores, viruses, fungi, and bacteria. Despite the advantages, its toxicity and flammability make it difficult to work with as a sterilant.
The typical methods for EO sterilization are two-fold: the gas chamber method and the micro-dose method. The gas chamber method benefits from economies of scale in that a large chamber is typically flooded with a combination of EO and other gases used as dilutants, as EO becomes highly flammable past 3% concentration. Drawbacks of this method include the air contamination produced by the fluorocarbon dilutants, EO residuals on materials (condensation), and, most prominently in this instance, the nonexistent portability of this method.
The micro-dose method is a more reliable and economically feasible version of the gas chamber method. It stipulates the use of a bag containing a pure EO-liquid-filled ampoule that, upon activation, evaporates and diffuses through the containing bag into a second bag that contains the items to be sterilized. Once there, contact sterilization occurs at a specified temperature and humidity. Unfortunately, the specifications on temperature and humidity are required to prevent excess condensation of the EO from occurring. This psychrometric limitation would prevent the use of this method in the field, unless a temperature and humidity controller could be added to the system.
NO2
NO2 is a highly effective and rapid sterilant for use against a spectrum of common bacteria, viruses, and spores. The qualities that diminish EO’s usage due to thermodynamic limitations do not inhibit NO2 (i.e., it can operate in a closed environment at room temperature and ambient pressure). The lethality mechanism for the substance is the DNA destruction of the biological agent via nitration of the phosphate backbone, which kills the organism as it absorbs NO2. This DNA degradation occurs at extremely low concentrations of the gas and carries with it a low boiling point, which likewise predicates that it will not condense in the majority of field operating environments. This fact implies that NO2, unlike EO-based processes, requires no aeration to remove condensation from the work piece immediately following the sterilization cycle. It is likewise less corrosive and compatible with most medical materials and adhesives. In addition, because NO2 does not diffuse as deeply into a material as EO does, the total sterilization and aeration time is significantly reduced from days to minutes or even less. Furthermore, the rapid aeration process allows for fewer residues on materials, and the residues themselves are not carcinogenic, cytotoxic, or teratogenic. This notion implies that materials can be handled immediately after the cycle has completed. Exhaust is handled by scrubbers, which are landfill-safe after saturation, side-stepping the hazardous materials limitations.
ADVANCED PROSTHETICS
Prosthetics have endured a significant evolution over the last two centuries. Unfortunately, field amputations were all too common across the 19th century battlefronts. Even worse, during this era, the afflicted lacked adequate means of anesthetic and were forced to endure crude, painful, and often fatal measures at preserving life. The medical community at the time was aware of this fact, but the technology simply did not exist for a higher quality of care.
Fast forward to the 20th century, and suddenly a different picture begins to emerge. Electronics are made small. Transistors the width of a hair strand no longer seem curious to the layman. And what follows (beginning in the late 1970s) is a systemic and grand transition of technological evolution in the area of miniaturized electronics.
Although the 1970s was not the genesis decade for the great advancements in prosthetics we see today, this period did lay the foundation upon which the field built much of its applied technologies—namely, advancement in applied controls, miniaturized motors, and biological sensing modalities. At a glance, these technologies may appear unrelated, but together they formed the core of advanced prosthetics in the modern age.
Control systems are nothing new to engineering; however, the applications by which control theory can be applied are ever evolving. In particular, the field of biologic sensing is incredibly young and only beginning to be understood and explored. And its participation in the field of prosthetics is obvious: people are not machines; they are biologic entities that behave like machines. Their movements are guided by predictable kinematics, their musculature systems are similarly driven by a series of cables and hinges, and the signals that these pieces of biologic machinery receive are electrical in nature. Unfortunately, the analogy pretty much ends there. Where a pure machine signal is discrete in nature, a biological marker of a similar indication is highly nonlinear and unpredictable in a human subject.
Nonetheless, this fact has not stopped research from pressing forward, especially in the northeastern United States. Two universities—Johns Hopkins and the University of Pittsburgh—in collaboration through DARPA-funded efforts, have achieved great success through extended programs. These two programs are known as the Revolutionizing Prosthetics program (RPI, RPII, and RPIII [note that the numbers represent different phases of funding]) and the Hand Proprioception and Touch Interfaces (HAPTIX) program. Further up the chain, these dollars come from the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative, which is sponsored by the White House to revolutionize the understanding of the brain and accelerate the development of new associated biological technologies.
In the field of neurologically controlled prosthetics (neuroprosthetics), when it comes to assessing biological data streams from the nervous system [5], two parameters are typically considered in obtaining the data: richness and invasiveness.
Richness describes how distinct a sensing modality is at representing a movement or motion. Invasiveness represents the qualitative measure of the ease by which a sensing platform may be applied and removed. The institutions of academia appear to be much more focused upon richness, and the limit of invasiveness has not yet been reached.
For example, spinal columns, as well as grey matter in the brain, have been tapped into, and direct nerve innervations have all been tested with varying degrees of success. These elements represent high invasiveness. On the other end of the spectrum is the array of pressure sensors, myoelectrics, and superficial electroencephalograms (EEGs), which do not penetrate the skin of the subject patient. These approaches fall under low invasiveness tactics.
Similarly, richness has its own spectrum. In the beginnings of neuroprosthetics (under the guidance of Dr. Andy Schwartz at the University of Pittsburgh), signals were noisy and poorly understood. In addition, repeated testing for the same action often yielded wildly different results, which can be attributed to the newness of the field as well as the poor understanding of the technology interface. It thus took many years of data analysis and pattern recognition algorithms to finally achieve a sense of how the brain and muscle system interact [6].
The relationship between richness and invasiveness is often one of direct proportion; increase one, and the other rises along with it. This phenomenon was observed in Dr. Schwartz’s trials. While invasiveness in itself is not a bad quality for a sensing modality, it does come with its drawbacks. Most noteworthy is biocompatibility. This factor is related to how organic tissues and their associated immune system responses interact with the implanted material. A good analogy is that of organ transplantation. If everything is not just so, the host body will attack and destroy the newly implanted organ. In the instance of implanted sensing modalities, the most common result of this interaction between tissue and nonorganic material is scar tissue, which can lead to signal degradation and attenuation over time. Currently, the field has a razor-sharp focus on the science of understanding these sensing modalities rather than on the engineering of enhancing their long-term viability, accuracy, and biocompatibility.
It serves the investigation, due to the high variety in technologies and limb geometries/kinematics, to compartmentalize the approach such that upper limb (UL) and lower limb (LL) prosthetics are considered separately. Statistically speaking, the LL amputation is much more prevalent due to the combat association with the amputation procedure. Many more Warfighters returning from the battlefield are LL amputees [7]. UL amputations tend to be congenital in nature, and so users are typically more adapted to using their residual limb as a non-weight-bearing tool. Likewise, these amputations occur at varying lengths relative to another part of the body. With LL, they are measured with respect to the hip, and with UL, they are measured with respect to the shoulder. This notion breaks the types into several categories. LL types include above the knee (AK), below the knee (BK), and hip disarticulation. UL types include humeral, radial, and shoulder disarticulation.
That being said, consider the potential cost associated with a Warfighter discharged to injury via limb amputation. This cost includes a lifetime of care through the U.S. Department of Veterans Affairs (VA); loss of mobility, dignity, and independence; a wide assortment of potential negative psychological effects directly tied to the injury itself (e.g., post-traumatic stress disorder [PTSD]); and a Warfighter unable to assist in forward operating efforts. This last notion, if addressed by advanced prosthetics, would supply the Warfigher with re-captured independence, dexterity, and mobility; a greater psychological state of health afforded by a restored limb; and a significantly reduced cost per soldier to the VA office. By having an advanced restored limb, many or all of the negative cascading effects associated with enduring amputation could be negated.
Parallel but separate efforts in the field of advanced prosthetics have been ongoing since the late 2000s. What is interesting to note, however, is how unique these efforts are for UL and LL prosthetics. The geometries and kinematics associated with the human leg and the arm are vastly different. Virtually no identical technology can be applied from one side of the gap to the other. Accordingly, their applied technologies and involved industries/ research efforts are distinct.
Three companies that specialize in advanced LL prosthetics are Otto Bock (Germany), Össur (Iceland), and Hangar Clinics (the United States). Each of these companies tended to develop at the outset of a war, where the local citizenry had a high prevalence of amputation. Accordingly, these companies have had their doors open for approximately a century or longer and have expanded their services beyond prosthetics (mostly into simplifying various disability scenarios). Typically, their research is conducted in house, and their technologies are proprietary. And collectively, most of their 21st century advances can be seen through computer-controlled knees and ankles, as well as unique flexible foot geometries to replicate a natural human gait.
Computer-controlled knees (such as that pictured in Figure 6) feature hydraulic systems that adjust the pressure of a fluid within the joints of the knee, providing the patient with counterweight, natural feel, and balance. These elements are critical for providing a stable platform on which the wounded Warfighter can rely in athletic scenarios. Similarly, computer-controlled ankles providing increasingly more degrees of freedom and platforms with greater foot flexion further enhance the natural feel of the limb and serve to create greater proprioception for the Warfighter wearing the leg [8].
As their histories of development have shown, the companies focused on LL are much more likely to collaborate with endowment foundations than with government institutions. This trend is likely to continue into the foreseeable future and lead to similar LL technologies across all three companies with discrete unique instances of deviation, depending on which foundation or post-academia researchers are working with the company at the time. Furthermore, technologies are starting to take shape that emulate the sensing philosophy of the UL research community in addition to these big companies beginning to expand into UL territory.
UL research has a different approach. Sponsored mostly under the umbrella of the BRAIN Initiative, a great amount of research has come from within the academic community via government research grants, sponsored by directed agencies such as DARPA. The UL research focus is especially directed toward implementing the brain to control advanced prosthetic equipment. As mentioned, the HAPTIX and Revolutionizing Prosthetics programs are the strongest efforts on this front.
CONCLUSION
With all the technological advances occurring on the homefront, the time has come to bring battlefield medical care into the 21st century. What were once statistically fatal injuries can now be treated with advanced superficial wound care, internal hemostatic agents, portable sterilization, and advanced prosthetics to afford wounded Warfighters the opportunity to continue to live happy, healthy lives and, in some cases, return to defending their country. Collaborative research and large funding efforts in academic, industrial, and governmental spheres have begun to advance the state of technology toward human enhancement and extended longevity [9]. These platforms, which are mostly at stages between development and deployment, will require industrial efforts to scale up their usefulness, such as is being performed by DARPA and the National Institutes of Health (NIH) through the BRAIN Initiative. With further collaborative efforts on these fronts, these technologies will further preserve the livelihood and health of the forces fighting to defend their countries.
References:
- Raman, R., and R. Bashir. “Stereolithographic 3D Bioprinting for Biomedical Applications.” Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champagne, 2015.
- Moholkar, V. S., A. B. Pandit. “Bubble Behavior in Hydrodynamic Cavitation: Effect of Turbulence.” AIChE Journal, vol. 43, no. 6, pp. 1641-1648, 1997.
- Heinglein, A., and M. Gutierrez. “Sonochemistry and Sonoluminescence: Effects of External Pressure.” Journal of Phyical Chemistry, vol. 97, pp. 158-162, 1993.
- International Agency for Research on Cancer. “1,3-Butadiene, Ethylene Oxide and Vinyl Halides (Vinyl Fluoride, Vinyl Chloride and Vinyl Bromide).” Vol. 97 of IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Lyon, France, pp. 185-287, 2008.
- Atzori, M., et al. “Electromyograpy data for noninvasive naturally controlled robotic hand prostheses.” nature.com, 23 December 2014.
- Gazzoni, M., et al. “Quantifying forearm muscle activity during wrist and finger movements by means of multichannel electromyography.” PLoS ONE, vol. 9, no. 10, 7 October 2014.
- Fatone S. “Development of Subiscial Prosthetic Sockets with Vacuum-Assisted Suspension for Highly Active Persons with Transfemoral Amputations.” U.S. Army Medical Research and Materiel Command, Fort Detrick, MD, October 2014.
- Tucker, M. R., et al. “Control strategies for active lower limb extremity prosthetics and orthotics: A review.” Journal of Neuroengineering and Rehabilitations, vol. 12, no. 1, 2015.
- Stanhope, S. J. “The BADER Consortium, Annual Report.” U.S. Army Medical Research and Materiel Command, Fort Detrick, MD, October 2014.


